Joana Brígida Capela1, Inês Almeida1, Mariana Reis1, Erica Guerreiro2, Carla Mendonça3, Maria João Virtuoso1.
1Pediatrics Service, Hospital de Faro, Centro Hospitalar Universitário do Algarve, Faro, Portugal,
2Ophtalmology Service, Hospital de Faro, Centro Hospitalar Universitário do Algarve, Faro, Portugal,
3Neuropediatrics and Development Center, Hospital de Faro, Centro Hospitalar Universitário do Algarve, Faro, Portugal.
ADDRESS FOR CORRESPONDENCE
Joana Brígida Capela, Rua Leão Penedo, 8000-386, Faro, Portugal.
Email: jbcapela@chua.min-saude.pt | | Abstract | Introduction: Cat Scratch Disease (CSD) is a zoonotic infectious disease, typically caused by Bartonella henselae. The disease usually has a benign course, but systemic involvement may occur. CSD is the most common cause of neuroretinitis.
Case Report: We present a case of a 16-year-old male, with a positive family history of neurodegenerative diseases, frequent contact with stray cats and four-week visual symptoms. Serologies for Bartonella henselae were positive for both IgM and IgG. The fundoscopy showed a macular star, that is typical of neuroretinitis by Bartonella henselae. He was started on corticosteroid and antibiotic treatment for six weeks, with full recovery after six months.
Discussion: We report a rare case presentation, where the clinical history was essential to guide the investigation. In the face of a suspicion of severe neuroretinitis due to Bartonella henselae, early treatment should be initiated. Mild cases usually have spontaneous remission. | | | | Keywords | | Neuroretinitis, Bartonella henselae, Teenager | | | | Introduction | Cat Scratch Disease (CSD) is a zoonotic infectious disease, typically caused by Bartonella henselae (BH), an intracellular gram-negative bacterium.1 Cats are the natural reservoir of these bacteria and may infect humans through scratches, bites or licks near the conjunctiva.1 Most cases occur in children and adolescents and in immunocompromised individuals.2 The disease usually has a benign course, but systemic involvement may occur, even in immunocompetent patients.3
The most common presentation of CSD is a cutaneous lesion (nontender erythematous pustules or papules) at the sight of inoculation, that lasts for 3-10 days and associated self-limited regional lymphadenopathy.2,4 These may be combined with systemic symptoms such as fever, malaise, fatigue, anorexia, nausea, vomiting, weight loss, headache, night sweats, rash or arthralgia.1,3 In some cases, atypical presentations, like cutaneous, hepatosplenic, cardiac, renal, pulmonary, ophthalmological, neurological, hematologic and musculoskeletal manifestations may occur, but they are rare among immunocompetent patients (5-14%).3,5
Neuroretinitis is characterized by unilateral optic neuropathy, with inflammation of the peripapillary retina and optic nerve, resulting from a variety of bacterial, viral, fungal, parasitic and inflammatory etiologies.5 CSD is the most common cause of neuroretinitis, although it is rare (1-2% of the patients) and might affect immunocompetent children.6,7
Neuroretinitis can present with unilateral reduced visual acuity, afferent pupillary defect, visual field defect (including central scotoma) and dyschromatopsia.2,4 Ocular symptoms manifest 2-3 weeks after the onset of systemic symptoms, although they can appear in their absence.4 The referred pain on extraocular movements is not often described.7 On ophthalmology examination, fundoscopy findings are usually consistent with a swollen optic nerve and a macular star.3 Optic disc oedema decreases within two weeks, but complete resolution may take up to three months.1 OCT shows flattening of the fovea, a thickened neurosensory retina and subretinal fluid accumulation.7 The differential diagnosis includes other infectious diseases, inflammatory optic neuropathies, demyelinating, autoimmune, compressive and toxic conditions (Table 1).1,2,5,7
Table 1. Differential diagnosis of acute visual loss accompanied by optic disc swelling6
| Neurological or Ophthalmological conditions |
Malignancies or systemic conditions |
Infectious diseases |
| Optic neuritis/ neuropathy |
Intracranial malignancies |
Cat scratch disease |
| Leber hereditary optic neuropathy |
Paraneoplastic disorders |
Lyme disease |
| Intracranial hypertension |
Orbital tumors |
Syphilis |
| Optic disc drusen |
Nutricional and toxic optic neuropathy |
Tuberculosis |
| Retinal artery/ vein occlusion |
Sarcoidosis |
Salmollenosis |
| Uveitis/ chorioretinitis |
Behçet disease |
Leptospirosis |
| Diabetic papillopathy |
Severe high blood pressure |
Toxocariasis |
| |
|
Toxoplasmosis |
| Histoplasmosis |
| Viral diseases (mumps, varicella, rubella, measles, Epstain-Barr, Cytomegalovirus, Herpes simplex virus and Hepatitis B virus) |
Diagnosis of BH neuroretinitis includes clinical findings of young age and history of contact with cats, signs and symptoms of typical neuroretinitis (including positive fundoscopy and OCT findings), systemic symptoms and positive serology (IgG and IgM).1 IgM positivity for BH indicates acute or very recent infection and confirms the CSD diagnosis.1 If IgM is negative, the diagnosis is controversial, despite a positive IgG measurement.2 IgG level of 1:64 or less suggests absent active infection, while levels of 1:256 and above confirms acute infection.4 Nonetheless, levels between 1:64 and 1:256 suggest possible CSD and retesting after 10-14 days is mandatory.4 Blood culture, skin testing, lymph node biopsy and PCR are also among the confirmatory tests in patients with suspected CSD.2 The sensitivity of a PCR test in blood is low, so a negative result does not exclude the diagnosis.8
The treatment of atypical presentations of CSD is controversial. While mild cases can resolve spontaneously, antibiotic therapy may prevent serious complications and shorten the duration of symptoms in severe cases.3 Standardized treatment recommendations do not exist, so antibiotic recommendations differ for each clinical presentation.3 Neuroretinitis is typically a self-resolving condition, although treatment should be considered for high-risk patients (like immunocompromised children) or in patients with profound vision loss.5 The mainstay treatment normally includes steroids and antibiotic therapy, in most case reports with rifampicin in combination with doxycycline, for at least 2-6 weeks. Full recovery is usually achieved.3,7,8
| | | | Case Report | We present a case study of a 16-year-old male with a positive family history of glaucoma and neurodegenerative diseases, who lived in a rural area and had regular contact with farm animals, dogs and stray cats. The patient came to the emergency department with a four-week visual acuity loss in the right eye (RE), accompanied by retrobulbar pain, color vision distortion and a scotoma in the center of the visual field.
A few days prior to the onset of visual changes, he had flu-like symptoms, with myalgia and arthralgia. He referred having been in occasions scratched by cats. He denied having had fever, headache, vomiting, weight loss, night sweats, cough or other neurological symptoms.
On observation, he had a visual acuity of the RE 0,3 and subnormal color vision. Fundoscopy of the RE revealed papillary oedema and optic disc engorgement. No adenopathies or skin lesions were noted and the neurologic examination was normal. The computerized optical tomography (OCT) showed optic nerve changes (Figure 1) and maculopathy of the RE (Figure 2). He was admitted for further investigation and was started on methylprednisolone 30 mg/kg/day for five days, with only slight relief of the symptoms.
Figure 1. A. Right eye OCT (on the right) and left eye OCT (on the left), on admission. Right eye OCT shows disc edema, subretinal fluid accumulation and retinal thickening. B. Right eye OCT (upper figure) and left eye OCT (down figure), on admission. Right eye OCT reveals maculopathy, with retinal thickening and subretinal fluid.
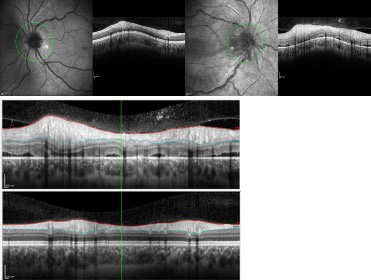
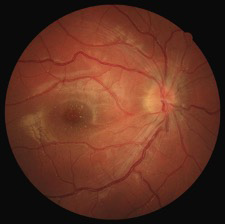
Figure 2. Fundoscopy with a macular star, characteristic of neuroretinitis by Bartonella henselae.
The white blood count, renal and hepatic function, C-reactive protein or sedimentation rate were unremarkable. Serologies for Bartonella henselae were positive for both IgM and IgG (IgM >32 and IgG >128). All the other serologies tested were negative (Mycoplasma pneumoniae, Cytomegalovirus (CMV), Human Immunodeficiency Virus (HIV) 1 and 2, Ebstein-Barr (EBV), Borrelia burgdorferi, Herpes simplex virus (HSV) 1 and 2, Varicella zoster virus (VZV) and Treponema pallidum). Mantoux, IGRA test, anti-MOG and anti-aquaporin-4 antibodies were also negative. For the cerebrospinal fluid (CSF), the cytochemical exam was normal and polymerase chain reaction (PCR) for neurotropic agents and bacteriologic culture were negative. CSF immunoelectrophoresis revealed bands with polyclonal IgG distribution (considered normal). The magnetic resonance imaging (MRI) of the brain, orbits and spinal cord did not show any relevant changes. Clinical aspects and positive serologies for Batonella henselae motivated antibiotic treatment with doxycycline 100 mg 12/12 hours and rifampicin 300 mg 12/12 hours.
Infection with Bartonella henselae was confirmed in a second sample taken two weeks later, with IgM 1:512 and IgG 1:4096. The ophthalmology exam was also repeated two weeks later and the fundoscopy showed a macular star, that is typical of neuroretinitis by Bartonella henselae (Figure 2). CSD with neuroretinitis was therefore confirmed and antibiotic therapy was continued for 6 weeks.
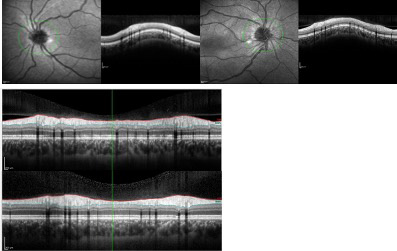
He was discharged with steroid therapy in a weaning regime. On fundoscopy, the macular star disappeared after two months and there was no papilledema or optic disc engorgement after three months. The OCT was normal after five months (Figure 3). Complete recovery of visual acuity, with normal visual field and color vision in the RE was achieved after six months.
Figure 3. A. Right eye OCT (on the right) and left eye OCT (on the left), five months later after admission. Right eye OCT shows full recovery. B. Right eye OCT (upper figure) and left eye OCT (down figure), five months after admission. Right eye OCT shows nearly complete resolution of maculopathy.
| | | | Discussion | We report a rare case presentation, where the clinical history was essential to guide the investigation. In our case report, the boy had three of the referred manifestations for neuroretinitis and he referred flu-like symptoms a few days before visual symptoms started. The macular star may be absent early in the disease, developing within 2 weeks, as in our case report and may last for up to one year; it is caused by leakage of lipid-rich exsudates due to increased permeability of the optic nerve capillaries.1,2,6
In the face of a suspicion of severe neuroretinitis due to Bartonella henselae, early treatment should be initiated, although there is not enough evidence that delay in starting therapy leads to incomplete remission or sequelae. Mild cases usually have spontaneous remission. In a retrospective multicenter study involving 86 patients, the outcomes appear to have been worse in the group that received antibiotic therapy alone compared to the group that received antibiotics and corticosteroids.9 The choice of antibiotic for Bartonella henselae infection depends on the site of infection, with doxycycline, rifampin, azithromycin, ciprofloxacin, trimethoprim-sulfamethoxazole and gentamicin being effective options.10 When there is central nervous system involvement, the combination of doxycycline and rifampin appears to be the most appropriate regimen, with good results and complete recovery and appropriate susceptibility without described resistances. However, the use of antibiotics is not universally agreed upon, as in other retrospective studies, the use of antibiotic therapy, with or without corticosteroids, did not seem to alter the prognosis.11 Empirical treatment with doxycycline 100 mg every 12 hours (2.2 mg/kg if <45 kg) and rifampin 10 mg/kg every 12 hours appears appropriate in immunocompromised or immunocompetent patients with moderate to severe systemic disease or significant vision loss.9 doxycycline can be replaced with azithromycin in children under eight years of age (due to the risk of irreversible dental discoloration with prolonged doxycycline treatment), at a dose of 500 mg every 24 hours and then 250 mg every 24 hours (if <45.5 kg, 10 mg/kg every 24 hours and then 5 mg/kg every 24 hours) and ciprofloxacin or trimethoprim-sulfamethoxazole are options in case of allergy.9 In the case of suspected infectious neuroretinitis without an identified cause, some authors recommend starting treatment with doxycycline and rifampin for four to six weeks, with coverage for Bartonella henselae, Lyme disease, Rocky Mountain spotted fever, leptospirosis and possible benefit in syphilis, in addition to good tolerance and good penetration into the central nervous system.9 Regarding corticosteroids, studies also do not concur on their benefit in vision recovery. Considering the morbidity associated with corticosteroids and the limited robust evidence for their use in neuroretinitis, routine systemic corticosteroid use is not recommended. However, due to their potent anti-inflammatory effect, they may theoretically offer benefit in severe cases with vision loss, fulminant optic disc edema or significant intraocular inflammation, in combination with antibiotic therapy over six weeks with gradual tapering.9,11 We chose the therapy described with good results and complete resolution of the clinical condition.
Regarding the ophthalmological follow-up, although follow-up protocols for cases of neuroretinitis caused by Bartonella henselae have not been identified in the literature, the authors consider it relevant to have regular assessments of visual acuity, fundoscopy and OCT every two or three weeks, to monitor the resolution of optic disc edema and macular star. Long-term considerations include monitoring visual function and early identification of any secondary complications, such as the development of corticosteroid-induced cataracts or disease recurrence every three months, until completing an 18-month period. After this period, annual ophthalmological evaluation may be recommended. Considering the family history of the teenager in question and the fact that some neurodegenerative diseases manifest with retrobulbar neuritis, it is crucial to have regular follow-up, with particular attention to acute vision changes. | | | | Compliance with Ethical Standards | | Funding None | | | | Conflict of Interest None | | |
- Ksiaa, I. et al. Update on Bartonella neuroretinitis. J. Curr. Ophthalmol. 31, 254-261 (2019). [CrossRef]
- Karti, O., Ataş, F. & Saatci, A. O. Posterior Segment Manifestations of Cat-scratch Disease: A Mini-review of the Clinical and Multi-modal Imaging Features. Neuro-Ophthalmology 45, 361-371 (2021). [CrossRef]
- Lemos, A. P., Domingues, R., Gouveia, C., de Sousa, R. & Brito, M. J. Atypical bartonellosis in children: What do we know? J. Paediatr. Child Health 57, 653-658 (2021). [CrossRef]
- Conde, E., Antueno, E., Palmieri, F. & Cheistwer, A. Neurorretinitis por Bartonella henselae en un adolescente. Arch. Argent. Pediatr. 119, 616-620 (2021). [CrossRef]
- Nikolic, B. et al. Child Neurology: Bartonella henselae Neuroretinitis in 2 Patients. Neurology 98, 896-900 (2022). [CrossRef]
- Jurja, S. et al. brain sciences The Clinical Profile of Cat-Scratch Disease's Neuro-Ophthalmological Effects. (2022). [CrossRef]
- Sykes, D. A. W., Joseph, S. L., Williams, S. P. & Das, S. U. A 13-Year-Old Girl With Unilateral Visual Changes. 0-3 (2023) doi:10.1177/23247096221150635. [CrossRef]
- Alonso Montejo, M. del M. & Muñoz Vilches, M. J. Bartonella henselae neuroretinitis: a case report. Med. Clin. (Barc). 157, 199-200 (2021). [CrossRef]
- Fairbanks, A. M., Starr, M. R., Chen, J. J. & Bhatti, M. T. Treatment Strategies for Neuroretinitis : Current Options and Emerging Therapies. (2019) doi:10.1007/s11940-019-0579-9. [CrossRef]
- Raihan, A. R., Zunaina, E., Wan-Hazabbah, W. H., Adil, H. & Lakana-Kumar, T. Neuroretinitis in ocular bartonellosis: A case series. Clin. Ophthalmol. 8, 1459-1466 (2014). [CrossRef]
- Cirone, D., Mandarà, E., de Simone, L., Pellegrini, F. & Cimino, L. Ophthalmic manifestations of cat scratch disease. Ann. Eye Sci. 6, 0-1 (2021). [CrossRef]
DOI: https://doi.org/10.7199/ped.oncall.2026.62
|
| Cite this article as: | | Capela J B, Almeida I, Reis M, Guerreiro E, Mendonça C, Virtuoso M J. Bartonella henselae neuroretinitis in a healthy teenage boy. Pediatr Oncall J. 2024 Feb 16. doi: 10.7199/ped.oncall.2026.62 |
|