Catarina Macedo Francisco1, Sara Vale2, Andreia Fernandes3, João Lameiras Campagnolo4, Mafalda Pires5.
1Pediatrics Department, Hospital Sousa Martins, Guarda, Portugal,
2Department of Pediatrics, Leiria Hospital, Portugal,
3Department of Pediatrics, ULS Algarve - Faro Unit, Portugal,
4Department of Orthopedics, Dona Estefânia Hospital, CHULC, Portugal,
5Department of Physical Medicine and Rehabilitation, Dona Estefânia Hospital, CHULC, Portugal.
ADDRESS FOR CORRESPONDENCE
Catarina Macedo Francisco, Morada: Rua José Afonso, nº7, 2º direito, 1600-130, Lisboa.
Email: acjfrancisco@hotmail.com | | Abstract | Background: Osteogenesis imperfecta (OI) is a rare heterogeneous connective tissue disorder, characterized by frequent fractures, progressive bone deformities, bone dysplasia, joint hypermobility and muscular weakness. It stands as the most prevalent genetic bone disorder, with an estimated prevalence ranging between 1 : 15 000 –20 000 births.
OI treatment main goal is to reduce fracture frequency, optimize bone alignment, increase the patient’s mobility and quality of life, reduce pain and control extra skeletal signs.
Methodology: Retrospective descriptive analytic study based on hospital data records of patients followed in our specialized outpatient clinics, with follow-up at our multidisciplinary bone fragility outpatient clinic from 1995 up to December 2023.
Descriptive and statistical analysis using Microsoft Excel® and SPSS® v.27.
Results: A total of 54 patients were eligible for study, of which 67% (n=36) were males. Regarding OI clinical types distribution: type I: 51.8%(n=28), type III: 20.3%(n=11) and type IV: 13%(n=7).
There was one deceased patient, with OI type III: 96% (n=52) presented fractures. Considering extra-skeletal presentations, blue sclera and dentinogenesis imperfecta were the most frequent.
Regarding pharmacological treatment with bisphosphonates, 25 patients underwent treatment with pamidronate, with a statistically significant reduction in the number of fractures (p=0,002).
Conclusion: OI is an extremely complex disease with great clinical variability and having a great impact on patients’ quality of life.
Bisphosphonates remain the mainstay of treatment of OI with satisfactory results mainly on the reduction of number of fractures, as shown in our study.
A multidisciplinary approach is proven to treat these patients adequately.
| | | | Keywords | | Osteogenesis imperfecta, Treatment, Pediatrics | | | | Introduction | Osteogenesis imperfecta (OI), also known as brittle bone disease, is a rare and genetically heterogeneous connective tissue disorder1, characterized by frequent bone fractures, progressive deformities of long bones, ribs and spine, bone dysplasia, joint hypermobility and muscular weakness.2 Main extra skeletal signs include dentinogenesis imperfecta, altered sclera color and conductive or neurosensory hearing impairments.2
It stands as the most prevalent genetic bone disorder, with an estimated prevalence ranging between 1 : 15 000 –20 000 births, although milder forms are probably underdiagnosed.3
Only 0.008% of the world’s population is affected by OI, with currently half a million patients worldwide.3 Considering this estimate, one might expect around 660 carriers of the disease in Portugal, although concrete data are not available.
The course of the disease can vary greatly, ranging from mild forms to perinatally fatal outcomes.2
In the past, OI was thought to be caused only by dominant mutations in the genes encoding type I collagen (COL1A1 and COL1A2) resulting in defective type I collagen and patients with OI were classified as Sillence types I-IV based on the clinical phenotype1 , with disease severity as follows: I < IV < III < II2.
Presently, 14 genetic mutations are described, involving a vast array of genes encoding within the collagen biosynthesis pathway or involved in osteoblast differentiation and bone mineralization4, with recognized mode of inheritance ranging from autosomal dominant (AD), autosomal recessive (AR) and X-linked recessive (XR) inheritance.1
From the clinical perspective, the phenotypic grouping remains very important and so, the International working group on constitutional diseases of bone, proposed a new classification, grouping OI into five types, according to genetic mutation and phenotype. Nevertheless, most centers use the old Sillence classification, in order to simplify OI groups.
As it is a rare and complex disease, it requires follow-up in a tertiary healthcare center in a multidisciplinary outpatient setting.
OI treatment depends on its severity, with the main goals being to reduce fracture frequency, optimize bone alignment, increase the patient’s mobility and independence, reduce pain, detect and control extra skeletal signs in a timely manner.4
It can be broadly divided into three main branches: medical treatment, rehabilitation and orthopedic surgery.
Regarding medical treatment, several strategies exist to slow down bone resorption and increase bone density. In an off-label manner, calcium and vitamin D supplementation is always done. Bisphosphonates are the next drug class more frequently used, as they inhibit the function of osteoclasts. Numerous studies have shown that bisphosphonates increase bone mineral density (BMD)4, although there is some controversy on this topic.
Other medical strategies used to address low bone mineral density and bone fragility5 are: monoclonal antibodies, (i.e.: denosumab), growth hormone, analogs of parathyroid hormone, anti-sclerostin antibodies, beta transforming growth factor inhibitor and gene therapy.2 Most of these modalities are options for adult patients only. Others are not available in our country.
Rehabilitation is another important basis of treatment, promoting developmental milestones attainment in early stages of life and functional recovery after fractures and/or surgeries.
Orthopedic surgery is a main pillar of OI treatment, because it focuses on the approach to acute fractures and also on the planned treatment of bone deformities and bone fragility secondary prevention.
Functional prognosis largely depends on OI type.6 The most significant indicators include the location and severity of bone deformities, bone mineral density and consequent frequency and severity of fractures.6
Our multidisciplinary outpatient clinic was created about 20 years ago and includes Orthopedics, Physiatry and Rheumatology. Patients with diagnosis or suspicion of OI are followed throughout the course of their disease until adult age.
The authors’ goals are to describe and characterize the pediatric population with OI followed in a tertiary hospital multidisciplinary outpatient clinic, evaluate the treatments performed and assess the clinical outcomes, namely fracture reduction and presence of bone deformities.
| | | | Methods & Materials | Retrospective descriptive analytic study based on hospital electronic data records of patients followed in bone fragility specialized outpatient clinic.
Demographic and clinical data was collected (gender, age at diagnosis, genetic and phenotypic type of OI, clinical manifestations (skeletal and extraskeletal), pharmacologic treatment modalities and timing, presence of bone deformities, number and type of surgeries performed).
Patient sample included children and adolescents with follow-up at our multidisciplinary bone fragility outpatient clinic from 1995 up to December 2023. Patients included were under 18 years of age and either had a definitive OI diagnosis or had positive clinical and family history and were under genetic testing at the time.
Patients who lost follow-up due to missed appointments were to be excluded from the study.
Statistical analysis was carried out using Microsoft Excel® and SPSS® v.27 .
Statistical significance was considered at a value of p<0.05 and to correlate variables, Wilcoxon test was used.
| | | | Results | A total of 54 patients were eligible for study, of which 67% (n=36) were males. The median age was four years old at the time of our study.
No patients were excluded from our study.
Regarding OI clinical types distribution: type I: 51.8%(n=28), type III: 20.3%(n=11) and type IV: 13%(n=7) (Figure 1).
Figure 1. OI types.
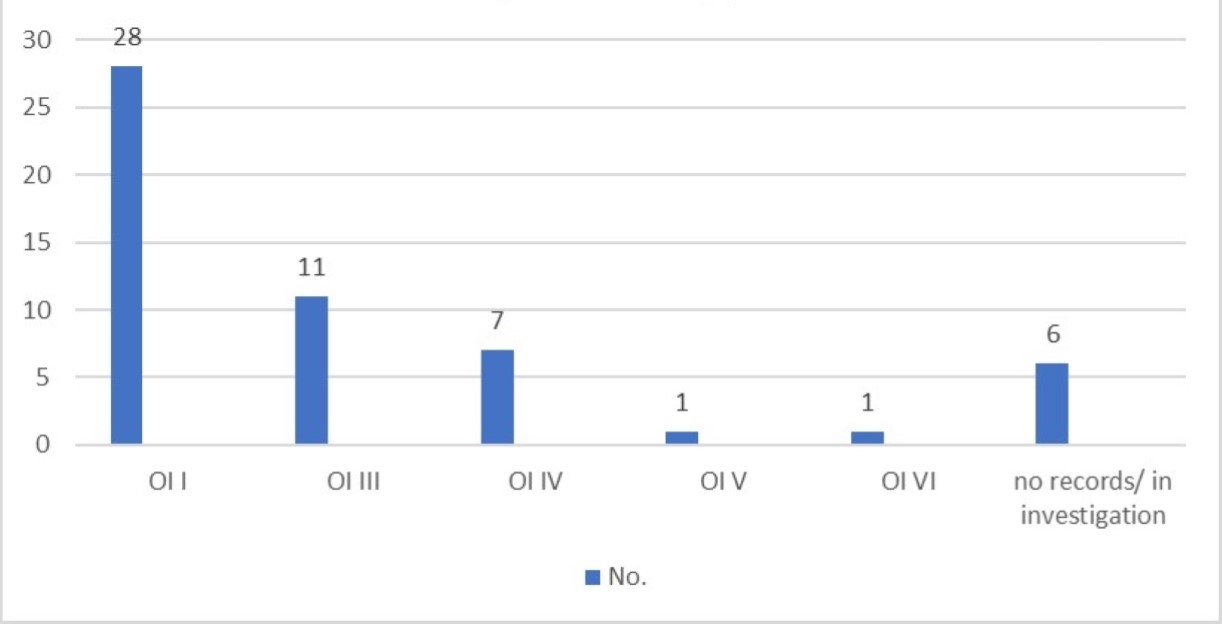
Median age at diagnosis was 4 years-old for type I OI, 5,4 years-old for type III and 7,4 years-old for type IV. Only one patient had prenatal diagnosis, revealing type I OI.
In six patients it was possible to identify COL1A1 mutation and COL1A2 was identified in four patients. In three patients, genetic testing is still ongoing. Other rarer mutations, such as WNT1 or PLOD2 mutation were also found, each in one patient. In the remaining patients (n=39), there was no mention of genetic mutation.
24 patients had family history of OI.
About 96% (n=52) presented fractures which were most common in lower limbs (n=43), followed by upper limbs (n=31) and only three patients had a history of vertebral fractures (Table 1).
Considering extra-skeletal clinical presentations, 25 patients presented blue sclera and 14 had dentinogenesis imperfecta. Four patients had hypoacusia, two had ligamentous hyperlaxity, four had triangular facies and two had microcephaly (Figure 2).
Figure 2. OI Symptoms.
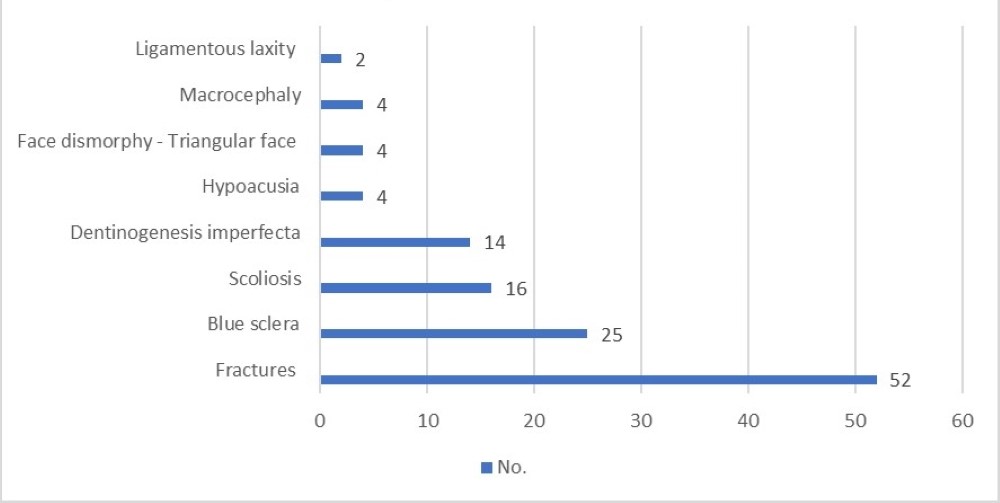
Table 1. fracture sites
| Fracture sites |
No. |
| Lower limbs |
43 |
| Upper limbs |
31 |
| Clavicle |
5 |
| Vertebral bodies |
3 |
| Costal fractures |
2 |
| Pelvic fractures |
1 |
Median number of fractures was 4 in OI type I patients, 6 in OI type III and 4 in type IV.
About 30% (n=16) of patients had scoliosis. Of these, 37,5% were type I OI patients, 25% were OI type IV patients and 18,6% were type III OI patients.
Regarding other bone deformities, upper limb deformities were present in 5 patients, all OI type III patients (Figure 3), which means these were present in 45% of OI type III patients.
Figure 3. Skeletal symptoms stratified by OI type.
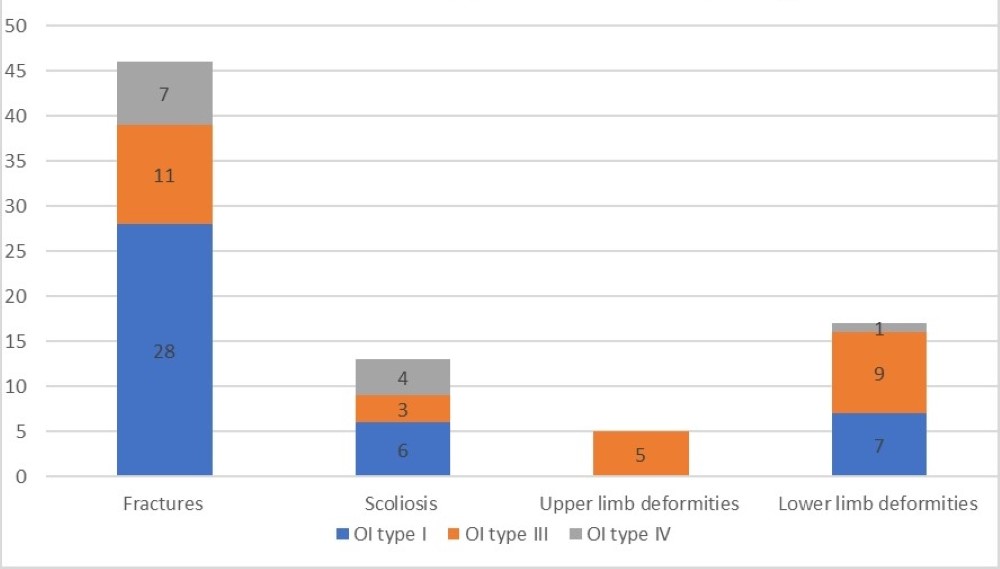
Lower limb deformities were found in 19 patients, with the majority, about 47%, being present in OI type III patients and about 37% in OI type I patients. This makes a total of 81.8% of OI type III patients having lower limb deformities. In type I OI they were present in only 25%.
Data regarding the total number of skeletal symptoms is presented in Figure 3.
Considering pharmacological treatment with bisphosphonates, 24 patients underwent treatment with pamidronate, three with zoledronate and one with pamidronate and alendronate combined. The average age at the beginning of treatment was 2.25 years-old and average duration of treatment was 3.4 years.
Longest average treatment duration was 4.8 years for OI type III patients, followed by OI type IV, with 2.9 years, with OI type I patients with an average treatment duration of 2.5 years.
All of the 25 patients had fractures prior to the bisphosphonate treatment, with an average of 3.08 fractures per patient.
After bisphosphonate treatment, only six patients had additional fractures, with an average of 3.1 fractures per patient up to the present time.
For patients who started and completed bisphosphonate treatment (n=20), the characterization of the number of fractures before and after treatment is presented on Figure 4. The authors compared the difference between the number of fractures before and during the treatment and after treatment completion. These results show a statistical significance with a - p value of 0,002, using Wilcoxon test (Table 2).
Figure 4. Comparison of number of fractures during the course of bisphosphonate treatment.
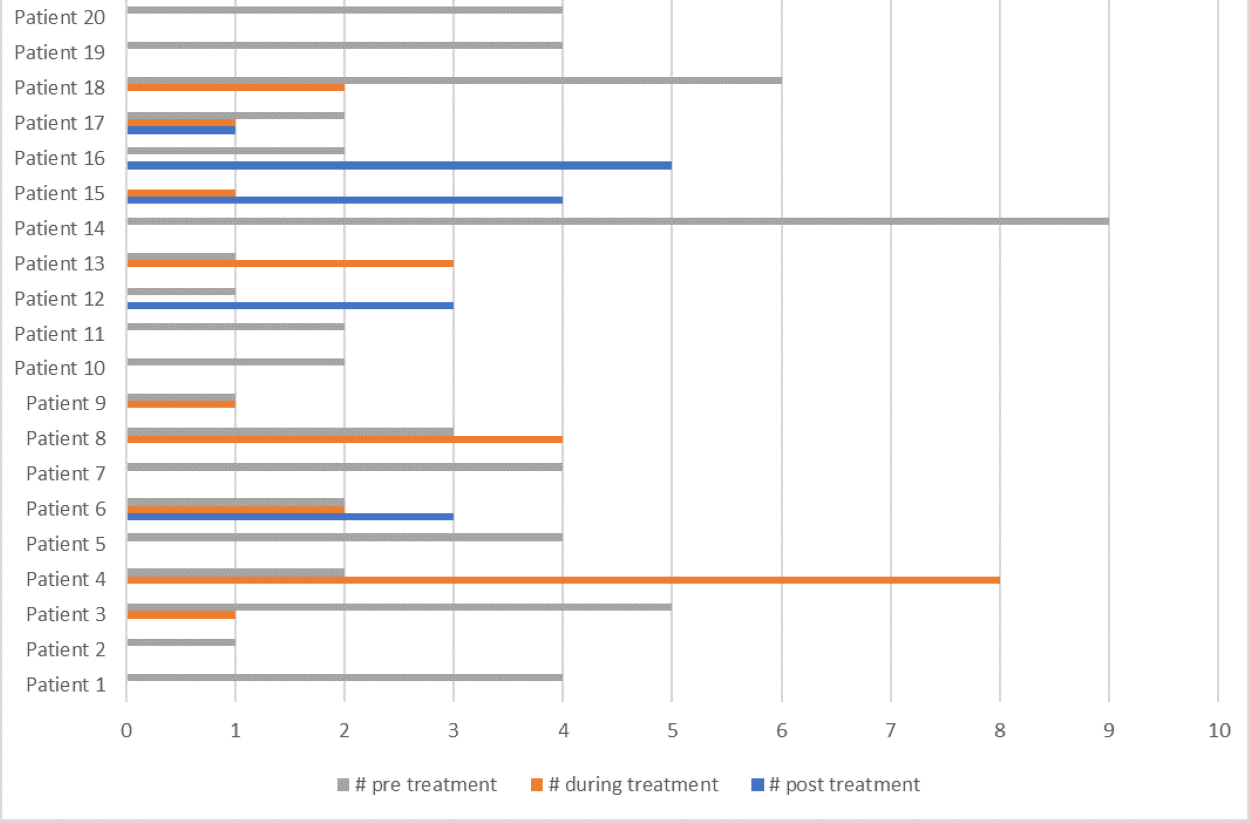
Table 2. Wilcoxon test, SPSS statistics.
| N |
No. of fractures before treatment (average) |
No. of fractures after treatment (average) |
p |
| 20 |
4,1 |
0,8 |
0,002 |
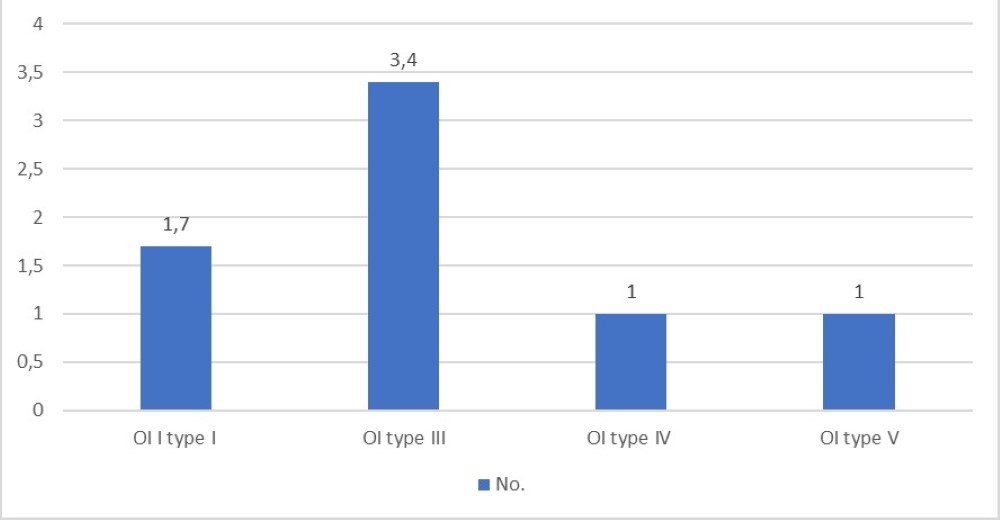
When considering surgical intervention, elongating intramedullary rods were used in five patients, internal fixation nails in nine patients and Kirschner wires in two patients. In 15% of the patients (n=8), more than one surgery was necessary
Of these, five patients had type I and six had type III OI. Average number of fractures per patient are displayed in Figure 5.
The maximum number of surgeries per patient was six, in one patient with OI type III.
Figure 5. Average number of surgeries per patient.
| | | | Discussion | Our study, to our knowledge, is the largest sample of OI pediatric patients in our country so far (n=54).4,7
There was a higher prevalence in males (67%), which is not in accordance with what's described in the literature, that states there is no gender predominance, as the inheritance pattern is not gender-dependent.1,2,5
Regarding the type of OI, the most prevalent was type I, which is in accordance with the literature.1,2,6
Fractures were the most common symptom in OI patients with long bones of arms and legs being the most common places, which is in accordance with the literature.7
The number of fractures was higher in patients with type I and type III OI. Type III had the highest number of limb deformities, which seems logical to the authors, when considering the higher severity of the disease.2
The authors believe that vertebral fractures might be underdiagnosed due to its subtle presentation, which might explain the low number of vertebral fractures (n=3) in our study.
The most serious OI complication is platybasia, with consequent foramen magnum stenosis, more commonly occurring in type V OI.8 In our sample, no patient presented this symptom.
In our center, the most widely used bisphosphonate was pamidronate, with zoledronate and alendronate being less used.
Although our sample was rather small, bisphosphonates use showed a statistically significant reduction in the number of fractures, with a small number of patients having fractures after having completed the treatment.
Bisphosphonate therapy has been found to have a positive impact on vertebral morphology, including remodeling of deformed vertebrae in older children and preservation of vertebral shape when started early in life.9
This shows the dramatic improvement bisphosphonates have in OI, with a positive impact on the number of fractures, ultimately improving patients’ quality of life.
In the literature, bisphosphonates have been proven to be more effective in children than in adults and have a proven effect in increasing bone mineral density, although the reduction in the number of fractures was not yet verified.2
OI patients are administered with bisphosphonates both intravenously and orally. The advantages of intravenous administration are the possibility of dose titration, better bioavailability and absence of side effects in the gastrointestinal tract.2
The consumption of healthcare resources significantly decreases with the reduced number of fractures, resulting in fewer hospital visits and a great reduction in healthcare costs.
In our opinion, it would be of great interest to compare the efficacy of different bisphosphonates. Nevertheless, as this is a rather recent treatment, only in the future will it be possible to compare this data.
Regarding the OI surgical approach, 22 patients underwent surgery.
The literature describes the use of different osteosynthesis methods according to the need and expected outcome. For fracture prevention, intramedullary nailing of long bones in OI patients improves the quality of life predominantly by increasing mobility. The combined use of bisphosphonate and surgery allows for better outcomes.2
In our sample, internal fixation nails seem to be the preferred choice (n=9) and the most effective treatment, once it helps the bone elongation and the prevention of further fractures.
One of the major complications of this approach is nail migration4, but that didn’t occur in our sample.
These patients are complex, with a significant number needing more than one surgery, which, once again, reflects the high burden on healthcare that OI represents.
The authors acknowledge limitations to this study, with the main ones being: the classification as a retrospective study with data based on medical records with consequent lapses of information which limits data interpretation; and the small sample size, which does not allow for OI-Portuguese-population extrapolation. Nonetheless, this study gives a satisfactory characterization of OI patients and shows the success of different treatments implemented.
A factor that wasn’t explored by the authors was the OI effect on the quality of life of these patients. Meta-analysis exploring this topic shows that OI has a great impact on patients' quality of life, especially on the emotional, social and school levels.10
This impact is higher in adolescents, compared to children. OI type I was shown to be the type with the strongest impact on patients' mental health.10 Some strategies to improve this would be psychological counseling during the appointments and helping OI patients to cope with their disease. It would be interesting to conduct these types of studies in our hospital.
Related to this matter, it is also the presence of pain, how it’s perceived by OI patients and how they deal with it, something the authors didn’t approach and would be interesting to do. To better describe the pain experience of these patients, future research should focus on better characterizing OI pain with the use of age-appropriate valid, reliable and multidimensional pain assessment tools.11
| | | | Conclusion | OI is a complex disease with great clinical variability and significant impact on patients’ quality of life. Follow-up by a multidisciplinary team is essential.
Most common OI type is type I. All OI types were diagnosed during childhood, with OI type I diagnosis being made earlier than in other types.
Regarding symptoms, most common were bone fractures, present in all groups.
OI type III appears to have higher risk of fractures, higher prevalence of bone deformities and a longer treatment duration, which makes up for its severity. This is in accordance with what's described in the literature, that shows lower percentages of mobility in patients who have OI type III.12
As for treatment, bisphosphonates remain the mainstay of treatment of OI with satisfactory results mainly on the reduction of number of fractures, as shown in our study.
Nevertheless, as previously mentioned, new strategies are being explored, such as sclerostin inhibitory antibodies and TGF beta inhibition, to address not only the low bone mineral density but also inherent bone fragility.
A multidisciplinary approach, with a combination of medical treatment, rehabilitation and surgery, when needed, is proven to treat these patients adequately.
It’s important to address patients’ quality of life and studies showing its impact on daily activities are necessary, in order to target the main factors to be improved when treating these patients.
| | | | Compliance with Ethical Standards | | Funding None | | | | Conflict of Interest None | | |
- Yu, H., Li, C., Wu, H. et al.: Pathogenic mechanisms of osteogenesis imperfecta, evidence for classification. Orphanet J Rare Dis 18. 234. [CrossRef] [PubMed] [PMC free article]
- Burtsev M.E., Frolov A.V., Logvinov A.N., Ilyin D.O., Korolev A.V: Current approach to diagnosis and treatment of children with osteogenesis imperfecta // Pediatric Traumatology, Orthopaedics and Reconstructive Surgery. 2019, 7:87-102. 10.17816/PTORS7287-102. [CrossRef]
- Forlino A, Cabral WA, Barnes AM, Marini JC: New perspectives on osteogenesis imperfecta. Nat Ver Endocrinol. 2011:540-557. 10.1038/nrendo.2011.81. [CrossRef] [PubMed] [PMC free article]
- Escobar C, Malveiro D, Salgado A, Santos MI, Lameirão Campagnolo J, Cassiano Neves M: Osteogénese imperfeita--experiência do serviço de. 2013:5-11. 10.20344/amp.4005. [CrossRef] [PubMed]
- Marom R, Rabenhorst BM, Morello R.: Osteogenesis imperfecta: an update on clinical features and therapies. Eur J Endocrinol. 2020, 183:95-106. 10.1530/EJE-20-0299. [CrossRef] [PubMed] [PMC free article]
- Subramanian S, Anastasopoulou C, Viswanathan VK: Osteogenesis Imperfecta. [Updated 2023 Feb 6]. In. StatPearls [Internet, Treasure Island (FL): StatPearls Publishing; 2023.
- Sousa Martins, R. , Baptista, P., Rocha, S., Guimas, A. e Ribeiro, R. 2022: Osteogénese Imperfeita Em Adultos: A Experiência de um Centro Hospitalar. Medicina Interna. 29, 2 (Jun. 2022:120-126. 10.24950/rspmi.631.
- Ludwig K, Seiltgens C, Ibba A, Saran N, Ouellet JA, Glorieux F, Rauch F: Craniocervical abnormalities in osteogenesis imperfecta type V. Osteoporos Int. 2022, 33:177-183. 10.1007/s00198-021-06088-x. [CrossRef] [PubMed]
- Jeanne M. Franzone, Suken A. Shah, Maegen J. Wallace, Richard W: Kruse, Osteogenesis Imperfecta: A Pediatric Orthopedic Perspective, Orthopedic Clinics of North America, Volume 50, Issue 2. 2019:193-209. 10.1016/j.ocl.2018.10.003. [CrossRef] [PubMed]
- Wehrli, S., Rohrbach, M. & Landolt, M.A: Quality of life of pediatric and adult individuals with osteogenesis imperfecta: a meta-analysis. Orphanet J Rare Dis 18. 123. [CrossRef] [PubMed] [PMC free article]
- Nghiem, Tracy BSc, MSc (A)*; Louli, Julie BSc, MSc (A)*; Treherne, Stephanie C. BSc, MSc (A)*; Anderson, Charlotte E. BSc, MSc (A)*; Tsimicalis, Argerie RN, PhD*,†; Lalloo, Chitra BHSc, PhD; Stinson, Jennifer N: RN-EN, PhD, CPN; Thorstad, Kelly MSc(A)N, PHCNP. Pain Experiences of Children and Adolescents With Osteogenesis Imperfecta: An Integrative Review. The Clinical Journal of Pain 33(3): p 271-280, March. 2017. 10.1097/AJP.0000000000000395.
- Sawamura, Kenta MDa; Kitoh, Hiroshi MD, PhDa,b,*; Kaneko, Hiroshi MD: PhDa; Kitamura, Akiko MDa; Hattori, Tadashi MD, PhDa. Prognostic factors for mobility in children with osteogenesis imperfecta. Medicine 101(36): p e30521, September. 092022, 10.1097/MD.0000000000030521. [CrossRef] [PubMed] [PMC free article]
DOI: https://doi.org/10.7199/ped.oncall.2025.46
|
| Cite this article as: | | Francisco C M, Vale S, Fernandes A, Campagnolo J L, Pires M. Osteogenesis imperfecta - Experience of a Portuguese healthcare center. Pediatr Oncall J. 2024 Apr 23. doi: 10.7199/ped.oncall.2025.46 |
|